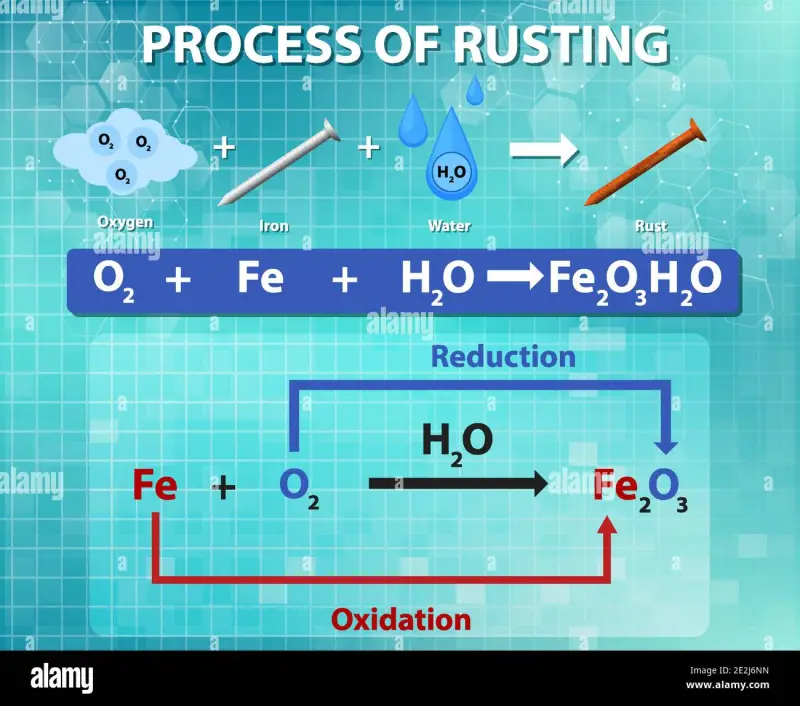
Rust is a commonly known term used to describe the oxidation of iron. Rust is a chemical compound, scientifically known as iron(III) oxide (Fe2O3). It is formed when iron reacts with water and oxygen in the presence of air. This reaction can happen to iron-based metal objects that are exposed to the environment, leading to their deterioration and corrosion over time.
Iron oxide is a red-brown colored compound that forms on the surface of iron and steel objects that are exposed to moisture and air. As rust continues to form, it will eventually flake off, exposing more of the metal to the elements, which leads to further corrosion. This can be a problem for objects made of iron and steel, as it weakens the material and makes it more susceptible to breakage.
It is possible to prevent or slow down the formation of rust by applying protective coatings to metal objects or keeping them stored in a dry and cool place. In addition, by removing rust and repairing the damaged metal, the object can be protected from further corrosion and deterioration. However, once rust has formed, it can be difficult to remove, and the only solution is often to replace the object entirely.
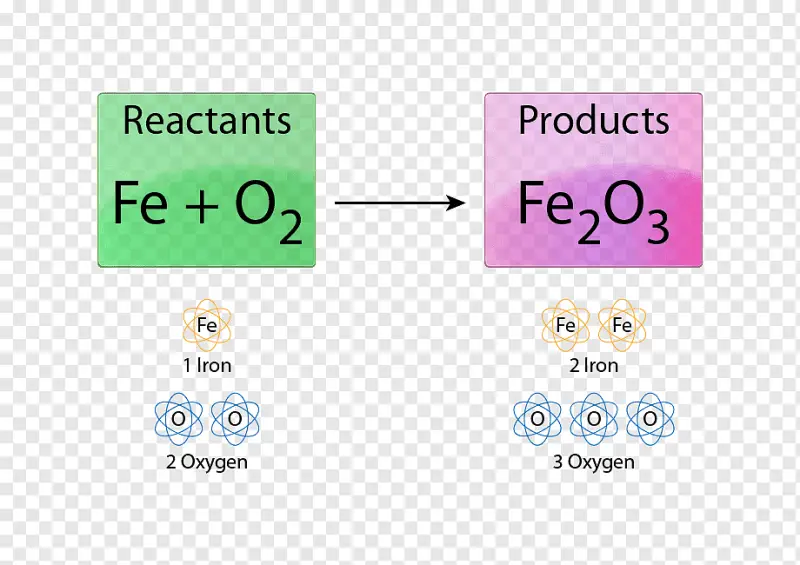
Chemical Formula For Rust Overview
You see, when iron reacts with water and oxygen in the presence of air, the chemical reaction results in the formation of iron(III) oxide. This reaction is commonly referred to as rusting, and it is what causes iron and steel objects to deteriorate and corrode over time.
The beauty of rust is that it’s so easily recognizable, thanks to its red-brown color. This color forms on the surface of iron and steel objects when they are exposed to moisture and air. As rust continues to form, it will eventually flake off, exposing more of the metal to the elements, which leads to further corrosion. This process can be quite damaging to metal objects, as it weakens the material and makes it more susceptible to breakage.
However, there are many amazing products, methods, and materials available that can help prevent or slow down the formation of rust. For example, applying protective coatings to metal objects or storing them in a dry and cool place can help prevent rust formation. And, if rust has already formed, removing it and repairing the damaged metal can help protect the object from further corrosion and deterioration.
The iron(III) oxide (Fe2O3) formula is such a fascinating example of how chemical reactions can result in the formation of new compounds, and how these compounds can impact the materials around us.
Comparative Table of Ingredient for the Chemical Formula of Rust
The chemical formula for rust, iron(III) oxide (Fe2O3), consists of three main ingredients: iron (Fe), oxygen (O), and water (H2O). Let’s take a closer look at each ingredient and compare their properties.
- Iron (Fe): Iron is a silvery-white metal that is abundant in the earth’s crust. It is a key component in the production of steel and is commonly used in construction, transportation, and other industries. Iron is also an essential element for the human body, as it is necessary for the production of hemoglobin.
- Oxygen (O): Oxygen is a colorless, odorless gas that makes up about 21% of the earth’s atmosphere. It is essential for human life, as we breathe in oxygen to support our metabolism. Oxygen also plays a crucial role in the formation of rust, as it reacts with iron to form iron(III) oxide.
- Water (H2O): Water is a colorless, tasteless liquid that is essential for life. It is a solvent, which means that it dissolves other substances and helps facilitate chemical reactions. In the case of rust formation, water acts as a medium for the reaction between iron and oxygen, and it also helps to promote the formation of iron(III) oxide.
| Ingredient | Properties |
|---|---|
| Iron (Fe) | Silvery-white metal, abundant in earth’s crust, used in construction, transportation, and other industries, essential element for human body |
| Oxygen (O) | Colorless, odorless gas, makes up 21% of earth’s atmosphere, essential for human life, crucial for rust formation |
| Water (H2O) | Colorless, tasteless liquid, essential for life, acts as a solvent and helps facilitate chemical reactions in rust formation |
In conclusion, each ingredient in the chemical formula for rust, iron(III) oxide (Fe2O3), plays a crucial role in the formation of rust. Iron provides the source material, oxygen reacts with iron to form iron(III) oxide, and water acts as a medium for the reaction and helps to promote rust formation.
Equipment To Work With Chemical Formula For Rust
When working with the chemical formula for rust, iron(III) oxide (Fe2O3), it is important to have the proper equipment to ensure safety and accurate results. Here’s a table of equipment that can be used when working with the chemical formula for rust:
| Equipment | Description |
|---|---|
| Safety goggles | Protect the eyes from any flying particles or splashes |
| Gloves | Protect the hands from any chemicals or reactions |
| Lab coat | Protect clothing from any spills or splashes |
| Face mask | Protect from inhaling any chemicals or fumes |
| Measuring beakers | Accurately measure and mix chemicals |
| Stirring rod | Mix chemicals and solutions |
| Dropper or pipette | Dispense small amounts of liquids accurately |
| Hot plate or Bunsen burner | Heat solutions and chemicals |
| Glassware set | Includes test tubes, flasks, and other containers to hold solutions |
It is important to follow safety protocols and guidelines when working with any chemicals, including the chemical formula for rust. Additionally, it is recommended to consult a laboratory safety manual or a supervisor for specific safety protocols and guidelines.
Step By Step Instruction On How To Make Chemical Formula For Rust
- Gather materials: You will need iron filings or shavings, hydrogen peroxide (H2O2), and a beaker or flask.
- Prepare the iron filings or shavings: Crush or grind the iron writings or shavings into a fine powder to increase their surface area.
- Add hydrogen peroxide: Pour the hydrogen peroxide into the beaker or flask. The amount of hydrogen peroxide used will depend on the amount of iron filings or shavings being used.
- Add the iron powder: Slowly add the iron powder to the hydrogen peroxide. Stir the mixture gently to ensure the iron is evenly dispersed throughout the solution.
- Heat the mixture: Place the beaker or flask on a hot plate or Bunsen burner and heat the mixture to boiling. Boil the mixture for 10-15 minutes.
- Observe the reaction: Over time, you will notice a brownish-red precipitate forming at the bottom of the beaker or flask. This is the rust, iron(III) oxide (Fe2O3), forming.
- Collect the rust: Once the reaction has completed, remove the beaker or flask from heat and allow it to cool. The rust can then be collected by filtering the solution through filter paper or a centrifuge.
- Dry the rust: Once collected, the rust can be dried by spreading it out on a piece of paper or a drying rack.
It is important to note that this process is a simplified version of the formation of rust and that rust typically forms through a more complex process involving exposure to air and moisture. This procedure is meant for demonstration purposes only and should not be used for any practical applications.
F.A.Q.
What is the chemical formula for rust?
The chemical formula for rust is iron(III) oxide, represented as Fe2O3.
What is the process of rust formation?
Rust formation is an oxidation process that occurs when iron reacts with water and oxygen to form iron(III) oxide.
Is rust harmful?
In general, rust is not considered harmful to human health. However, breathing in rust particles or dust can be irritating to the respiratory system and skin contact with rust can cause skin irritation.
How can rust be removed?
Rust can be removed through various methods, including sanding, scraping, wire brushing, and chemical rust removers.
Can rust be prevented?
Yes, rust can be prevented through protective coatings, such as paint or rust inhibitors, and by storing metal objects in a dry place to prevent exposure to moisture and oxygen.
What are the properties of rust?
Rust is a reddish-brown, flaky powder that is formed from the oxidation of iron. It is a poor conductor of electricity and has a low melting point.
Is rust magnetic?
No, rust is not magnetic. The magnetic properties of iron are lost when it reacts with water and oxygen to form rust.



Leave a Reply