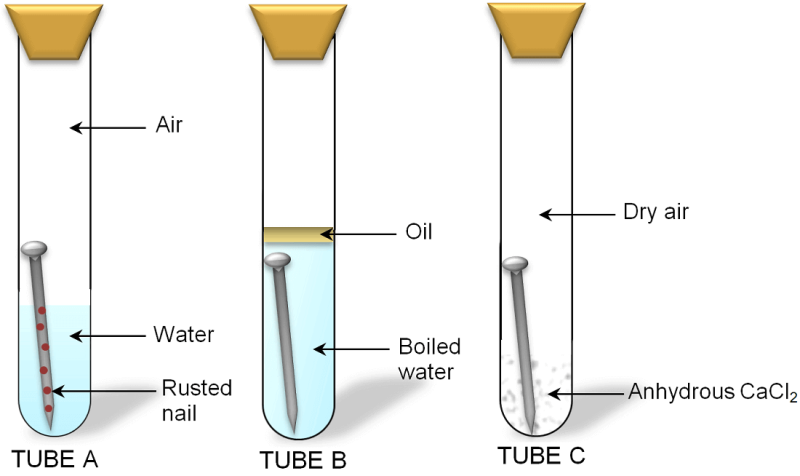
Rusting is a common form of corrosion that affects iron and steel objects. It occurs when iron reacts with water and oxygen, causing the iron to form iron oxide, also known as rust. Rusting can weaken the structure of metal objects and cause them to deteriorate over time, making them less durable and less functional. To prevent rusting, it is important to understand the conditions that lead to its formation.
One of the key conditions for rusting is the presence of moisture, such as water or humidity. The moisture provides the environment necessary for the reaction between iron, water, and oxygen to occur. The more moisture that is present, the faster the rusting process will be. Additionally, the temperature can also play a role in the rate of rusting, as higher temperatures can increase the rate of reaction.
Another important factor in rusting is the presence of air. The oxygen in air reacts with the iron to form rust, so areas with higher levels of oxygen will experience rusting faster than those with lower levels of oxygen. This is why metal objects that are stored in sealed containers or covered with rust-inhibiting materials are less likely to rust. By controlling these key conditions, it is possible to slow down or prevent rusting, helping to extend the life and durability of metal objects.
Conditions For Rusting Overview
First and foremost, you need moisture. This can be in the form of water, humidity, or even moisture in the air. The moisture provides the environment for the reaction between iron, water, and oxygen to occur, and the more moisture that’s present, the faster the rusting process will be. This is why it’s so important to protect metal objects from exposure to moisture, especially in coastal regions where the humidity levels are high.
Another important factor is the presence of air. Oxygen in the air reacts with the iron to form rust, so the more oxygen that’s available, the faster the rusting process will be. This is why metal objects that are stored in sealed containers or covered with rust-inhibiting materials are less likely to rust. And let me tell you, there are some fantastic rust inhibitors on the market today that are highly effective at slowing down or even stopping rust formation!
Finally, temperature also plays a role in the rate of rusting. Higher temperatures can increase the rate of reaction, so it’s important to be mindful of this when storing metal objects in hot climates. But, on the flip side, colder temperatures can slow down the rusting process, so in some cases, it may be beneficial to store metal objects in colder environments.
All in all, rusting is a complex and fascinating process that requires careful consideration of various conditions. But with the right products, materials, and methods, it’s possible to slow down or even prevent rusting and keep metal objects in top condition for years to come!
Conditions For Rusting Comparison Table
- Iron: Iron is the main element that reacts to form rust. Iron is present in many metal objects, including steel and cast iron. When exposed to the right conditions, iron reacts with water and oxygen to form iron oxide, or rust.
- Water: Water is a critical ingredient in the rusting process as it provides the environment for the reaction to occur. The more water that’s present, the faster the rusting process will be. This is why metal objects that are exposed to high levels of moisture, such as those in coastal regions, are more likely to rust.
- Oxygen: Oxygen in the air reacts with the iron to form rust. The more oxygen that’s available, the faster the rusting process will be. This is why metal objects that are stored in sealed containers or covered with rust-inhibiting materials are less likely to rust.
| Ingredient | Impact on Rusting |
|---|---|
| Iron | Essential for rust formation |
| Water | Increases the rate of rusting |
| Oxygen | Increases the rate of rusting |
It’s important to note that all three ingredients need to be present for rusting to occur. By controlling the presence of one or more of these ingredients, it’s possible to slow down or prevent rusting. For example, by keeping metal objects dry and away from air, it’s possible to significantly slow down the rusting process.
Equipment To Work With Conditions For Rusting
| Equipment | Purpose |
|---|---|
| Moisture meter | Measures the level of moisture in the air or on a surface, allowing you to monitor conditions that may lead to rusting |
| Rust inhibitor spray | Applies a rust-inhibiting coating to metal objects, helping to prevent rust formation |
| Dehumidifier | Reduces the level of humidity in the air, slowing down the rusting process |
| Rust dissolver | Dissolves rust, allowing you to clean and prepare metal surfaces for painting or other treatments |
| Paint or powder coating equipment | Applies a protective coating to metal objects, helping to prevent rust formation |
| Protective storage containers | Keeps metal objects dry and away from air, slowing down the rusting process |
Step By Step Instruction On How To Make Conditions For Rusting
To make conditions for rusting, you’ll need the following materials:
- Iron object (such as a nail or piece of steel)
- Water
- Air (or oxygen)
- A container to hold the object (such as a glass jar)
Guide on how to make conditions for rusting:
- Place the iron object into the container.
- Add water to the container, making sure that the iron object is completely submerged.
- Seal the container so that the iron object is completely enclosed and there is no exchange of air between the inside and outside of the container.
- Leave the container in a warm and humid location for several days.
- Observe the iron object and check for the formation of rust.
By following these steps, you should be able to create conditions for rusting and observe the formation of rust on the iron object. It’s important to note that the speed of rust formation will depend on a number of factors, including the temperature, humidity, and oxygen levels, as well as the type of iron object you’re using. But by following this basic procedure, you should be able to create conditions that will promote rusting.

F.A.Q.
What is rusting?
Rusting is a chemical process that occurs when iron reacts with water and oxygen to form iron oxide, also known as rust. Rusting can cause metal objects to weaken and deteriorate over time.
What are the three conditions for rusting?
The three conditions for rusting are iron, water, and oxygen. All three ingredients must be present for rusting to occur.
What happens when iron reacts with water and oxygen?
When iron reacts with water and oxygen, it forms iron oxide, or rust. This reaction can cause metal objects to weaken and deteriorate over time.
How can you prevent rusting?
You can prevent rusting by controlling the presence of water, oxygen, or both. For example, you can keep metal objects dry, cover them with rust-inhibiting materials, or store them in sealed containers.
How can you remove rust from metal objects?
You can remove rust from metal objects using a rust dissolver or abrasive materials, such as sandpaper or steel wool. You can also paint or powder coat the metal object to prevent rust from forming again in the future.
Is rust always harmful to metal objects?
Rust can weaken metal objects and cause them to deteriorate over time. In some cases, rust may also cause metal objects to fail completely. However, rust can also provide a protective coating for metal objects and prevent further corrosion.


Leave a Reply